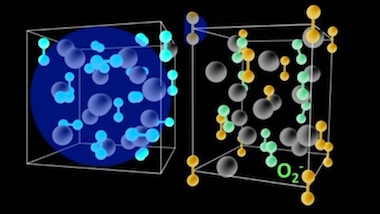
One of the very first attempts to understand the charge dynamics in mixed-valence systems dates back to 1939 when Evert Verwey, a Dutch chemist, observed a sudden jump in resistivity near -150 °C in magnetite. In the journal Science Advances now a research team of scientists from Germany and Slovenia reports a Verwey-type transition in a completely different class of mixed-valence compounds, which is composed of negatively charged dioxygen molecules. The compound Cs4O6 undergoes a phase transition from a state with indistinguishable molecular O2x- entities to a state with well-defined superoxide O2- and peroxide O22-anions. The breakthrough of the present study is the observation of such charge ordering in a simple crystal structure where novel physical phenomena is expected to emerge from intertwining of degrees of freedom pertinent to electronically active oxygen molecular units.